A fast and agnostic method for bacterial genome-wide association studies: Bridging the gap between k-mers and genetic events
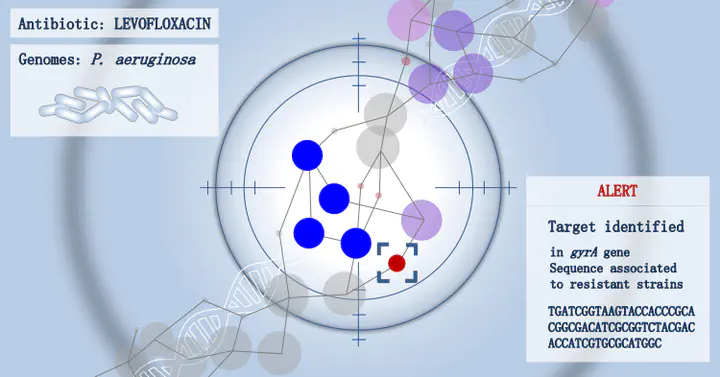 Image credit: Magali Jaillard
Image credit: Magali JaillardAbstract
Genome-wide association study (GWAS) methods applied to bacterial genomes have shown promising results for genetic marker discovery or detailed assessment of marker effect. Recently, alignment-free methods based on k-mer composition have proven their ability to explore the accessory genome. However, they lead to redundant descriptions and results which are sometimes hard to interpret. Here we introduce DBGWAS, an extended k-mer-based GWAS method producing interpretable genetic variants associated with distinct phenotypes. Relying on compacted De Bruijn graphs (cDBG), our method gathers cDBG nodes, identified by the association model, into subgraphs defined from their neighbourhood in the initial cDBG. DBGWAS is alignment-free and only requires a set of contigs and phenotypes. In particular, it does not require prior annotation or reference genomes. It produces subgraphs representing phenotype-associated genetic variants such as local polymorphisms and mobile genetic elements (MGE). It offers a graphical framework which helps interpret GWAS results. Importantly it is also computationally efficient—experiments took one hour and a half on average. We validated our method using antibiotic resistance phenotypes for three bacterial species. DBGWAS recovered known resistance determinants such as mutations in core genes in Mycobacterium tuberculosis, and genes acquired by horizontal transfer in Staphylococcus aureus and Pseudomonas aeruginosa—along with their MGE context. It also enabled us to formulate new hypotheses involving genetic variants not yet described in the antibiotic resistance literature.